Definition Of Isotopes In Scientific Terms
All non natural or man made elements are radioactive isotopes.
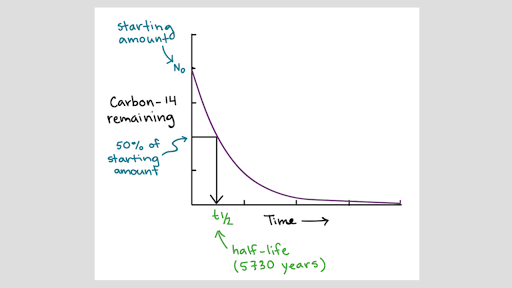
Definition of isotopes in scientific terms. The number of protons for different isotopes of an element does not change. Isotope definition any of two or more forms of a chemical element having the same number of protons in the nucleus or the same atomic number but having different numbers of neutrons in the nucleus or different atomic weights. Radioactive isotopes undergo decay. Isotopes are forms of an element whose nuclei have the same atomic numberâ the number of protons in the nucleus but different mass numbers because they contain different numbers of neutrons.
Many elements only exist in an unstable or radioactive form. Stable isotopes either never decay or else decay very slowly. The element with the most stable isotopes is tin which has ten different stable isotopes. The nucleus eventually reaches a stable number of protons and neutrons through one or more radioactive decays.
Heavier isotopes tend to react more slowly than lighter isotopes of the same element. Radioactive isotopes are commonly used in science industry and medicine. Isotopes of an element that have an unstable nucleus. Approximately 3 700 natural and artificial radioisotopes have been.
Isotopes are samples of an element with different numbers of neutrons in their atoms. Not all isotopes are radioactive.